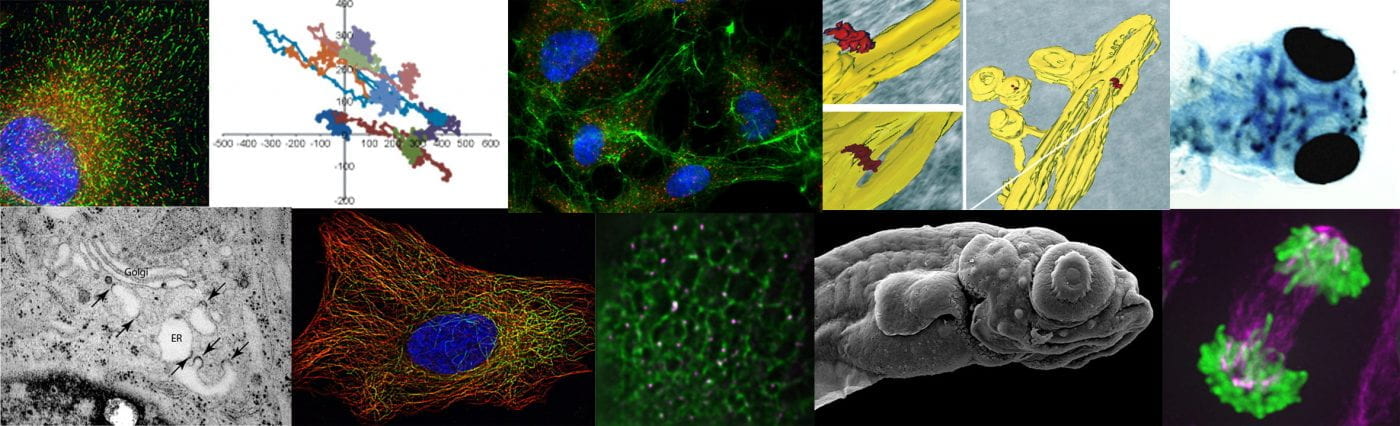
We take an integrated approach to membrane dynamics (“from nano- to Danio”) concentrating in the first instances on the biogenesis of transport carriers (including vesicles) and how membrane traffic is integrated with the cytoskeleton. We continue to use mammalian cell culture to define gene function using RNAi and CRISPR/Cas9 gene editing as well as through the use of GFP-tagging and live cell imaging to monitor and measure intracellular dynamics. Electron microscopy is a core technique for the lab to define the ultrastructural organization of cells during key transitions (e.g. cilia formation, collagen secretion).
Our goal is to understand intracellular patterning of cells: how is the internal architecture of a cell established and maintained and why is it organized in this particular way? The ER/Golgi interface provides an excellent angle to study this from with its unique layout of reticular ER, punctate ER exit sites, and juxtanuclear Golgi. Organization of these structures is intricately linked to the microtubule cytoskeleton and motor proteins. Our collaborative work on the endosomal system with Pete Cullen reinforces these ideas. We also aim to put all of our findings in the context of the healthy cell and organism which increasingly involves relating our more simple single cell tissue culture work to complex 3D organization up to the level of organs and even whole organisms.
1. The dynein-2 microtubule motor in ciliogenesis
Much of our recent work centres on the role of the dynein-2 motor in ciliogenesis. This is leading in exciting new directions and is now very much integrated as a core strand of our work. Our interests extend to the role of the secretory pathway in ciliogenesis and how the structure of the axoneme and specialized ciliary membrane form during the earliest stages of ciliogenesis. This of course has great significance to normal development as well as a cohort of human diseases, the ciliopathies. Our major questions here centre on the composition and function of the dynein-2 complex and how the early secretory pathway contributes to ciliogenesis. Our recent data showing that giantin and dynein-2 are linked functionally (Asante et al 2013) provides the underpinning data for this project. More recently we have defined the composition of the mammalian dynein-2 complex (Asante et al, 2014) and identified TCTEX1D2 as a candidate ciliopathy gene. This work provides the basis for us to understand how the subunits that are unique to dynein-2 participate in cargo delivery and transport within primary cilia. Our most recent work explores the role of the two dynein-2 intermediate chains, WDR34 and WDR60 using gene editing. Here, we find that they have apparently different roles in the complex and unexpectedly are required to establish and/or maintain ciliary transition zone organization (Vuolo et al., 2018).
Our major questions here centre on the composition and function of the dynein-2 complex and how the early secretory pathway contributes to ciliogenesis. Our recent data showing that giantin and dynein-2 are linked functionally (Asante et al 2013) provides the underpinning data for this project. More recently we have defined the composition of the mammalian dynein-2 complex (Asante et al, 2014) and identified TCTEX1D2 as a candidate ciliopathy gene. This work provides the basis for us to understand how the subunits that are unique to dynein-2 participate in cargo delivery and transport within primary cilia. Our most recent work explores the role of the two dynein-2 intermediate chains, WDR34 and WDR60 using gene editing. Here, we find that they have apparently different roles in the complex and unexpectedly are required to establish and/or maintain ciliary transition zone organization (Vuolo et al., 2018).
2. ER-to-Golgi transport: organization and function of ER export and the secretion of procollagen.
The COPII coat mediates selection of cargo and deformation of the ER membrane to generate transport vesicles. It is required for secretory cargo export from the ER and in its absence, cargo does not get transported to the Golgi. We are now investigating the role of TFG and Sec16 in early secretory pathway organization. This follows on from our work identifying and characterising Sec16 in human cells.
In metazoans COPII assembles at discrete sites called ER exit sites. We are investigating the mechanism by which this restricted assembly occurs and how this relates to the function of COPII. We have shown that the mammalian orthologue of Sec16 has a key role in this organization and our now investigating this is further detail. See our papers for further details. We are now extending out work to include electron microscopy (notably immunogold labelling of ultrathin cryosections and tomography, all in collaboration with Paul Verkade) and zebrafish genetics (in collaboration with Chrissy Hammond and Paul Martin). Here, we have made significant progress in recent years and are very keen to develop this work, especially exploiting the potential of zebrafish as a model organism. Our major question here is how organization of COPII-dependent budding relates to cell function. We also want to define how newly identified components of the system such as TFG direct the organization and function of ER exit sites. A particular focus of this work is how the organization of the early secretory pathway (ER, exit sites, ERGIC, and Golgi) relates to extracellular matrix secretion including of procollagen. Overall, this work focuses on how the internal structure of a mammalian cell relates to its functions. We are now developing this work in two specific directions: first, extending our long-standing interests in collagen transport and second, the organization and mechanisms of trafficking in neurons.
Our procollagen trafficking work has led us to challenge the prevailing model in the field that large COPII-coated carriers mediate the transfer of procollagen from the ER-to-Golgi. In live cell imaging using a newly developed procollagen reporter, we do not find evidence that such carriers make a significant contribution to trafficking. Instead, our data indicate that those exit sites in close proximity to the Golgi apparatus might be preferentially used.